Characteristics of low temperature lithium ion battery
1.At low temperatures, the viscosity of the electrolyte increases and the conductivity decreases;
2.The electrolyte/electrode interface membrane resistance and charge transfer resistance increase;
3.The migration rate of lithium ions in the active material body is reduced. As a result, the electrode polarization is increased at low temperatures and the charge and discharge capacity is reduced.
In addition, during low-temperature charging, especially during low-temperature high-rate charging, lithium metal precipitation and deposition will occur in the negative electrode. The deposited metal lithium is easy to irreversibly react with the electrolyte and consumes a large amount of electrolyte. At the same time, the thickness of the SEI film is further increased, leading to the battery The impedance of the negative electrode surface film further increases, and the battery polarization increases again, which will greatly damage the low-temperature performance, cycle life and safety performance of the battery.
This article discusses the modification methods used by researchers in recent years to improve the low-temperature performance of batteries from three aspects: positive electrode, electrolyte and negative electrode.
- Cathode material Cathode material is one of the key materials for the manufacture of lithium-ion batteries. Its performance directly affects various indicators of the battery, and the structure of the material has an important impact on the low-temperature performance of lithium-ion batteries.
The mainstream ways to improve the ion diffusion performance of cathode materials at low temperatures are:
- The method of surface coating the active material body with an excellent conductive material improves the conductivity of the cathode material interface, reduces the interface impedance, and reduces the side reaction of the cathode material and the electrolyte, and stabilizes the material structure.
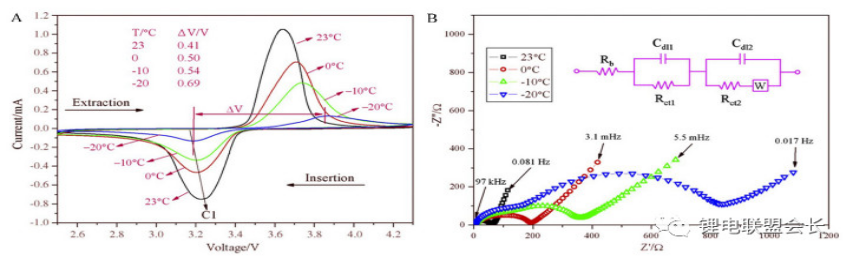
Rui et al. used cyclic voltammetry and AC impedance methods to study the low-temperature performance of carbon-coated LiFePO4, and found that the discharge capacity gradually decreases as the temperature decreases, and the capacity at -20°C is only 33% of the normal temperature capacity. The author believes that as the temperature decreases, the charge transfer impedance and the Weber impedance in the battery gradually increase, and the difference in the redox potential in the CV curve increases. This indicates that the diffusion of lithium ions in the material slows down at low temperatures, and the Faraday of the battery The weakening of the reaction kinetic rate caused a significant increase in polarization (Figure 1).

Figure 1 CV (A) and E IS (B) curves of LFP/C at different temperatures
- Bulk doping of the material body by Mn, Al, Cr, Mg, F and other elements, increasing the layer spacing of the material to increase the diffusion rate of Li+ in the body, reduce the diffusion resistance of Li+, and improve the low temperature performance of the battery .
Zeng et al. used Mn doping to prepare carbon-coated LiFePO4 cathode material. Compared with the original LiFePO4, its polarization at different temperatures is reduced to a certain extent, which significantly improves the electrochemical performance of the material at low temperatures. Li et al. doped the LiNi0.5Co0.2Mn0.3O2 material with Al and found that Al increases the interlayer spacing of the material, reduces the diffusion resistance of lithium ions in the material, and greatly increases the gram capacity at low temperatures.
- Reduce material particle size and shorten Li+ migration path. It should be pointed out that this method will increase the specific surface area of the material and increase the side reaction with the electrolyte.
Zhao et al. studied the effect of particle size on the low-temperature performance of carbon-coated LiFePO4 materials, and found that the discharge capacity of the material at -20°C increases with the decrease of the particle size. This is because the diffusion distance of lithium ions is shortened. The process of deintercalating lithium becomes easier. Sun and other studies have shown that the discharge performance of LiFePO4 decreases significantly with the decrease of temperature, and materials with small particle sizes have higher capacity and discharge platform.
- Electrolyte
As an important part of lithium-ion batteries, electrolyte not only determines the migration rate of Li+ in the liquid phase, but also participates in the formation of SEI film, which plays a key role in the performance of SEI film. At low temperatures, the viscosity of the electrolyte increases, the conductivity decreases, the impedance of the SEI film increases, and the compatibility with the positive and negative materials deteriorates, which greatly deteriorates the energy density and cycle performance of the battery.
At present, there are two ways to improve the low-temperature performance of the electrolyte: 1. Improve the low-temperature conductivity of the electrolyte by optimizing the composition of the solvent and using new electrolyte salts; 2. Using new additives to improve the properties of the SEI membrane to make it effective Conducive to Li+ conduction at low temperatures.
- Optimize solvent composition
The low-temperature performance of the electrolyte is mainly determined by its low-temperature eutectic point. If the melting point is too high, the electrolyte will easily crystallize out at low temperatures, which will seriously affect the conductivity of the electrolyte. Ethylene carbonate (EC) is the main solvent component of the electrolyte, but its melting point is 36°C, and its solubility in the electrolyte decreases or even precipitates at low temperatures, which has a greater impact on the low-temperature performance of the battery. By adding low melting point and low viscosity components to reduce the solvent EC content, the viscosity and eutectic point of the electrolyte at low temperatures can be effectively reduced, and the conductivity of the electrolyte can be improved.
Kasprzyk et al. obtained amorphous electrolyte by mixing two solvents of EC and poly(ethylene glycol) dimethyl ether. Only a glass transition temperature point appeared near -90°C. This amorphous electrolyte was extremely Dadi improves the performance of the electrolyte at low temperatures; at -60°C, its conductivity can still reach 0.014mS·cm-1, which provides a good solution for the use of lithium-ion batteries at extremely low temperatures.
2 New electrolyte salt
Electrolyte salt is one of the important components of electrolyte, and it is also a key factor to obtain excellent low temperature performance. At present, the commercial electrolyte salt is lithium hexafluorophosphate, and the formed SEI film has a large impedance, resulting in poor low-temperature performance. The development of a new type of lithium salt is imminent. Lithium tetrafluoroborate has a small anion radius, is easy to associate, and has a lower conductivity than LiPF6, but has a low charge transfer resistance at low temperatures, and has good low temperature performance as an electrolyte salt.
Zhang et al. used LiNiO2/graphite as the electrode material and found that the conductivity of LiBF4 at low temperatures is lower than that of LiPF6, but its capacity at -30°C is 86% of the capacity at room temperature, while LiPF6 based electrolyte is only 72% of the capacity at room temperature. This is due to the low charge transfer resistance of the LiBF4-based electrolyte and low polarization at low temperatures, so the low temperature performance of the battery is better. However, the LiBF4-based electrolyte cannot form a stable SEI film on the electrode interface, causing serious capacity degradation.
3 additives
The SEI film has a very important influence on the low temperature performance of the battery. It is an ionic conductor and an electronic insulator, and is a channel for Li+ to reach the electrode surface from the liquid phase. At low temperatures, the impedance of the SEI film increases, and the diffusion rate of Li+ in the SEI film decreases sharply, which deepens the accumulation of charges on the surface of the electrode, resulting in a decrease in the lithium insertion capacity of the graphite and an increase in polarization. By optimizing the composition and film-forming conditions of the SEI film, improving the ionic conductivity of the SEI film at low temperatures is beneficial to the improvement of the low-temperature performance of the battery. Therefore, the development of film-forming additives with excellent low-temperature performance is a current research hotspot.
Liu et al. studied the effect of FEC as an electrolyte additive on the low-temperature performance of the battery. The results show that the graphite/Li half-cell is at a low temperature of -20°C, and the electrolyte with 2% FEC is higher than the basic electrolyte at -20°C. The capacity increased by 50% during the first discharge, and the charging platform was reduced by about 0.2V. The XPS test shows that the SEI film formed by adding FEC electrolyte has a higher content of LiF than the SEI film formed by the electrolyte without FEC, which is conducive to the reduction of the impedance of the SEI film at low temperatures, thereby improving the battery’s performance. Low temperature performance.
- Anode material
The deterioration of the diffusion kinetics of lithium ions in the carbon anode material is the main reason that limits the low-temperature performance of lithium-ion batteries. Therefore, the electrochemical polarization of the anode is significantly increased during the charging process, which easily leads to the precipitation of metallic lithium on the surface of the anode.
Zinth et al. used neutron diffraction and other methods to conduct a detailed study on the lithium evolution behavior of the NMC111/graphite 18650 lithium-ion battery at a low temperature of -20°C. The battery is charged and discharged as shown in Figure 2, and Figure 3 is the Comparison of phase change of graphite negative electrode when charging at /30 and C/5 rates.

Figure 2 The relationship between the charge and discharge process Δ Q and time in the neutron diffraction experiment at a low temperature of -20 ° C

Figure 3 Comparison of phase changes of negative electrode after charging at different rates (A) and after 20h storage (B)
Choosing a suitable negative electrode material is a key factor in improving the low-temperature performance of the battery. Currently, the low-temperature performance is optimized mainly through methods such as negative electrode surface treatment, surface coating, doping to increase the layer spacing, and control of particle size.
1 Surface treatment Surface treatment includes surface oxidation and fluorination. Surface treatment can reduce the active sites on the graphite surface, reduce irreversible capacity loss, and at the same time generate more micro-nano structured pores, which is conducive to Li+ transmission and reduces impedance.
After the oxidation micro-expansion treatment, Zhang Lijin and others reduced the average grain size of graphite, and increased the amount of lithium ions embedded on the surface and edge of the carbon layer. The nano-scale pore structure introduced on the graphite surface further increased the lithium ion storage space. Wu et al. used 5at% fluorine gas to fluorinate natural graphite at 550°C, and the electrochemical performance and cycle performance of the treated material were greatly improved.
2 Surface coating Surface coating such as carbon coating and metal coating can not only avoid direct contact between the negative electrode and the electrolyte, improve the compatibility of the electrolyte and the negative electrode, but also increase the conductivity of graphite and provide more lithium insertion Site, so that the irreversible capacity is reduced. In addition, the layer spacing of soft carbon or hard carbon material is larger than that of graphite. Coating a layer of soft carbon or hard carbon material on the negative electrode facilitates the diffusion of lithium ions and reduces the resistance of the SEI film, thereby improving the low temperature performance of the battery. The surface coating of a small amount of Ag improves the conductivity of the negative electrode material, making it have excellent electrochemical performance at low temperatures.
The Fe/Fe3C-CNF composite material developed by Li et al. has good low-temperature performance and maintains a capacity of 250mAh·g-1 after 55 weeks of cycling at -5°C. Ohta et al. studied the influence of different negative electrode materials on the performance of lithium-ion batteries and found that whether it is carbon-coated artificial graphite or natural graphite, its irreversible capacity is greatly reduced compared with uncoated ones. At the same time, the carbon-coated graphite negative electrode can effectively improve the low-temperature performance of the battery. The discharge capacity retention rate of 5% coated graphite at -5°C is 90% of that at room temperature.
3 Increasing the gap between graphite layers The gap between the graphite anodes is small, and the diffusion rate of lithium ions between the graphite layers at low temperatures decreases, resulting in increased polarization. The introduction of B, N, S, K and other elements in the graphite preparation process can affect the graphite The structure is modified to increase the interlayer spacing of graphite and improve its ability to release/intercalate lithium. The atomic radius of P (0.106pm) is larger than that of C (0.077pm). P doping can increase the interlayer spacing of graphite and enhance the diffusion of lithium ions. At the same time, it is possible to increase the content of graphite crystallites in carbon materials. The introduction of K into the carbon material will form the intercalation compound KC8. When the potassium is removed, the interlayer spacing of the carbon material will increase, which is beneficial to the rapid insertion of lithium, thereby improving the low-temperature performance of the battery.
4 Controlling the negative electrode particle size Huang et al. studied the influence of negative electrode particle size on low-temperature performance, and found that coke negative electrodes with an average particle size of 6μm and 25μm have the same reversible charge and discharge capacity at room temperature, while at -30°C, the particle size The coke electrode with a diameter of 25μm can only release 10% of the room temperature capacity, and the coke electrode with a diameter of 6μm can release 61% of the room temperature capacity.
From this experimental result, it can be concluded that the larger the negative electrode particle size, the longer the lithium ion diffusion path and the greater the diffusion resistance, which leads to increased concentration polarization and poor low-temperature performance. Therefore, appropriately reducing the particle size of the negative electrode material can effectively shorten the migration distance of lithium ions between the graphite layers, reduce the diffusion resistance, increase the electrolyte infiltration area, and improve the low-temperature performance of the battery. In addition, the graphite negative electrode granulated by a small particle size single particle has higher isotropy, can provide more lithium insertion sites, reduce polarization, and can also significantly improve the low-temperature performance of the battery.
- Conclusion in summary
The low-temperature performance of lithium-ion batteries is a key factor restricting the application of lithium batteries. How to improve the low-temperature performance of lithium batteries is still a hot and difficult point of current research.
The battery system reaction process mainly includes four steps: Li+ transport in the electrolyte, crossing the electrolyte/electrode interface membrane, charge transfer, and Li+ diffusion in the active material body. At low temperatures, the rate of each step decreases, which causes the impedance of each step to increase, which leads to aggravation of electrode polarization, and causes problems such as reduction of low-temperature discharge capacity and lithium precipitation in the negative electrode.
To improve the low-temperature performance of lithium batteries, the influence of the positive electrode, negative electrode, electrolyte and other comprehensive factors in the battery should be comprehensively considered. By optimizing the composition of electrolyte solvent, additives and lithium salt, the conductivity of electrolyte should be improved, and the film formation resistance should be reduced. The polar material undergoes modification treatments such as doping, coating, and granulation to optimize the material structure and reduce the interface resistance and the diffusion resistance of Li+ in the active material body. Through the overall optimization of the battery system, the polarization of the lithium battery at low temperatures is reduced, and the low temperature

Leave A Comment